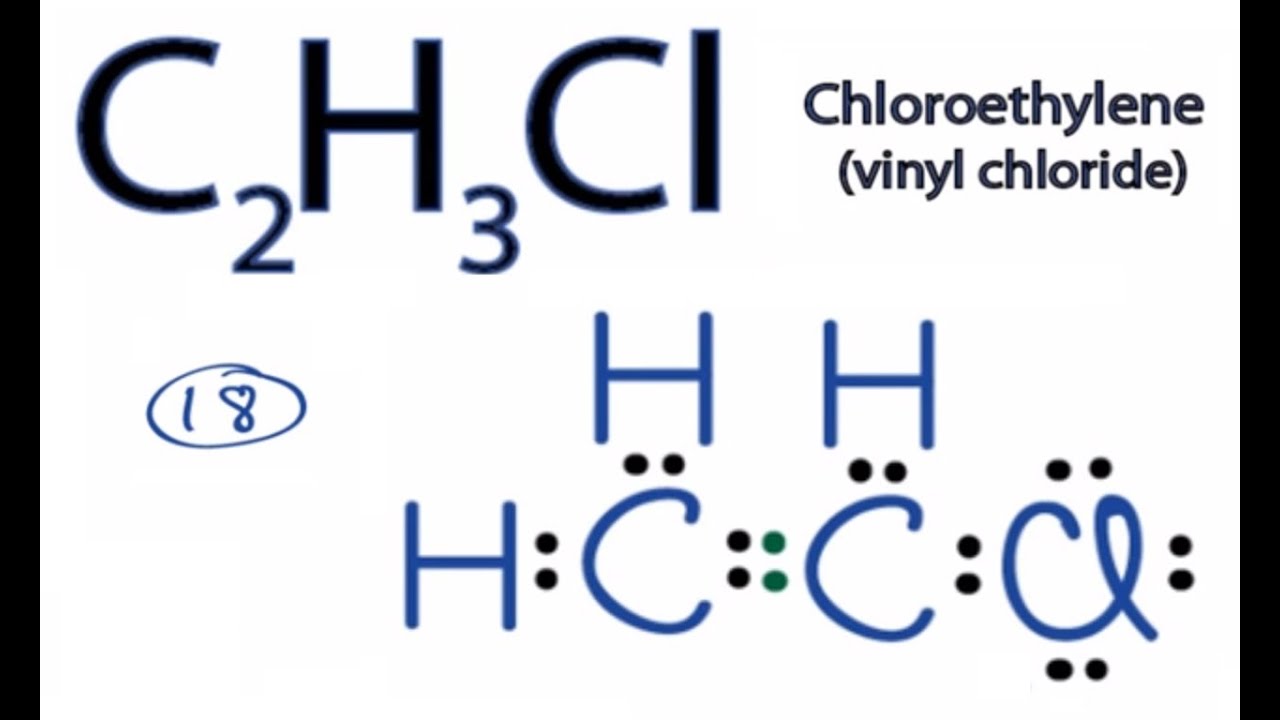
Draw a Lewis Structure for the Following Organic Molecule: C2h3cl
Chloroform is slightly polar in nature. B The repeating unit in polyvinyl chloride is OCH2OCHClO.

C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube
How to Draw the Lewis Dot Structure for C2H3Cl.

. The video covers the basic Lewis structures youll see in an introductory chemistry class. As we can see the 2 hydrogen atoms are covalently bonded to the oxygen atom which has two lone pairs 4 total electrons that push the. A nonflammable inhalation anesthetic.
Up to 256 cash back Draw the structure for the polymer produced from the monomer vinylidene chloride CH2-CCh. So we have C two H three C. The hybridization of chloroform is Sp³.
Log Octanol-Water Partition Coef SRC. A ammonia NH3 b water H2O c hydroniumion H3O. None of the atoms bears a formal charge and all atoms have octets except for hydrogen atoms which have duets.
Learn vocabulary terms and more with flashcards games and other study tools. Draw the Lewis structure for chloroacetylene C2HCI. Draw the Lewis structure for acetaldehyde C2H4O.
General considerations The structure needs to be assembled from the provided composition to comply with Lewis structure rules. Count up the total number of valence electrons and distribute them in such a way that every atom has an octet - remembering that 2 is an. Learn vocabulary terms and more with flashcards games and other study tools.
Vinyl chloride C2H3Cl differs from ethylene C2H4 in that one of the H atoms is replaced with a Cl atom. The bond angle of CHCl3 is 1095º. For the CH 3 Cl Lewis structure there are a total of 14 valence electrons available.
See explanation We start by looking at a water molecule. A C 2 H 3 Cl C C H Cl H H Solution. Classify the following hydrocarbon and draw a Lewis structure for it.
Start studying Organic Chemistry for Majors I Final Exam. Include all hydrogen atoms and show all unshared electron pairs. Place one electron pair between each pair of adjacent atoms as determined from the framework found in.
Problem 19 Convert the following molecular model of hexane a component of gasoline into a line-bond structure gray. Looking at the electronegativity and shape of the H_2O molecule tells you how the arrow depicts the polarity. Hexane 18 sp2 Hybrid Orbitals and the Structure of Ethylene.
From in between the hydrogen atoms to the oxygen atom. The net dipole moment of chloroform is 115 D. C2H5OH or Ethanol can simply be called or termed alcohol and it is an organic chemical compound.
Lewis Structure of O 2. Chloroethane is produced by hydrochlorination of ethylene. Draw a line-bond structure for propane CH3CH2CH3.
A compound might fit into more than one of the following classifications. Draw the structure of the copolymer produced from vinyl acetate and vinyl chloride. In this tutorial we are going to learn how to draw the lewis structure of C 2 H 2 step by step.
The molecular geometry of CHCl3 is tetrahedral. Draw the Lewis Dot Structure for the Hydrogen atom. The actual connectivity is H-O-N-O but you could also draw a reasonable Lewis structure for H-NO2 with formal charges on N and O After that you follow the normal procedure for drawing Lewis structures.
Draw a Lewis structure for C2H3CL. A give the bond order for the carbon carbon bond. 42 12 72 24 valence electrons.
1-2 Draw Lewis structures for the following compounds. Acetaldehyde molecules have one carbonyl group. All three fluorines are bonded to the same carbonc.
According to the octet rule a molecule should have eight electrons in its outer shell to become inert or stable. Lewis Structure example C2H3Cl C3H4 two. And chlorine essentially just acts as a hydrogen atom in terms of bond saturation.
1 Has the correct total number of valent electrons sum of valent electrons of component atoms adjusted for charge if it is an ion. The total valence electron available for drawing the chloroform CHCl3 lewis structure is 26. Start studying Organic Chemistry - Chapter 1.
Next draw lines between the atoms to represent that bond. Chloroethane commonly known as ethyl chloride is a chemical compound with chemical formula CH 3 CH 2 Cl once widely used in producing tetraethyllead a gasoline additive. A Draw the Lewis structure of vinyl chloride.
Draw the Lewis structure for the molecule. Plus Chlorine which is 7 and we have two Chlorines for a total of 24 valence electrons. Predict the value of each bond angle and indicate the overall shape of the molecule.
There are two structures with the formula C4H10. So if you have a compound with the Formula C2H4 this either indicates that you have this in this case since we only have two carbon atoms and not any more carbon atoms this indicates that we have a double bond in our structure. This geometry contains three C-Cl bonds and one C-H bond.
This copolymer is employed in some paints ad hesives and paper coatings Cl Vinyl Acetate Vinyl Chloride Isobutylene CH2-CCH32 is used to prepare cold. Vinyl chloride is used to prepare polyvinyl chloride which is an important polymer used in pipes. Vinyl chloride is an organochloride with the h 2 c chcl that also called the monomer of vinyl chloride VCM or chloreatenoThis colorless compound is an important industrial chemical mainly to produce Polymer polyvinyl chloride PVC.
Draw a Lewis structure for C2H3Cl. 143 Write a Lewis formula for each of the following organic molecules. Yeah so as a result of a double bond in.
Write a Lewis structure for each of the following organic moleculesa C2H3Cl vinyl chloride. Draw a Lewis structure for C2H3Cl. For cations subtract a number of electrons equal to the positive charge.
A video tutorial for how to draw Lewis Structures in five steps. Draw them and tell how they differ. Draw a Lewis Structure and classify each of the.
Draw both an electron-dot structure and a line-bond structure for vinyl chloride C2H3Cl the starting material from which PVC polyvinyl chloride plastic is made. Vinyl chloride is an organochloride with the h 2 c chcl that also called the monomer of vinyl chloride VCM or chloreatenoThis colorless compound is an important industrial chemical mainly to produce Polymer polyvinyl chloride PVC. Organic Chemistry 7th Edition Edit edition Solutions for Chapter 1 Problem 44P.
For anions add a number of electrons equal to the negative charge. Starting material for the preparation of poly vinyl chloride or PVC plasticsb C2HBrClF3 halothane.

Draw A Lewis Structure Of Formaldehyde Lewis College Life Hacks Middle School Science
C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube

Solved Write A Lewis Structure For Each Of The Following Organic Chegg Com
No comments for "Draw a Lewis Structure for the Following Organic Molecule: C2h3cl"
Post a Comment